Product Description
Matrix-511 Product Summary
Laminin 511 is known to bind to Intergin α6β1 of the cell surface. iMatrix-511 is highly purified human Laminin 511-E8 fragments recombinantly expressed by CHO-S cell.
Application
iMatrix-511 can be used as cell culture matrix for various cell types including ES/iPS cells.
Contents
Liquid Solution (175 μg/vial @ 0.5 mg/mL Solvent: PBS(-)) .
Product Size:
N-892011: 350 μg (175 μg x 2 pcs.)
N-892012: 1050 μg (175 μg x 6 pcs.)
Specifications
Format: Solution (175 μg/vial @ 0.5 mg/mL)
Storage temperature: 2-15°C
NaCl concentration: 137 mM
Activity
The dissociation constant of the binding activity with integrin α6β1 is under 10 nM.
Procedure
1. Dilute the solution with PBS (-). Coat dishes with 0.5 μg/cm². The optimal coating concentration depends on cell lines from 0.1 to 1.5 μg/cm². (For example, when you use 6-well plate (9.6 cm²/well), add 9.6 μl iMatrix-511 (4.8ug) in 1.99ml of PBS(-). Add 2 mL of diluted iMatrix-511 solution to the well.)
2. Incubate for 1 hour at 37°C, 3 hours at Room temperature, or over night at 4°C.
3. Remove excess fluid from the coated surface. No rinse is needed.
4. Immediately plate the cells at desired density.
Notes: Don't allow the plate to dry. Briefly spin down all liquid in the tube before use. Avoid repeated freeze-thaw cycles.
Documentation
Download Protocol [PDF]
Download MSDS [PDF]
Product Basics & Structure
iMatrix-511 is an innovative cell culture matrix compatible with a wide variety of cell types, and exceptionally well suited for pluripotent stem cells. This product is comprised of recombinant Laminin-511 E8 protein fragments which permit ES/iPS cells to be maintained in xeno-free culture conditions, enable the passaging of single cells, and provide greater adhesion than full-length Laminin, Vitronectin or Matrigel⁵.
Laminin-511 is a major component of the basement membrane used as a scaffold for pluripotent stem cells (ES/iPS cells), as it binds to integrin on cell surfaces. However Laminin-511 is a large protein (800kDa) composed of three chains (α-, β-, and γ-) forming a supramolecular aggregate, making it difficult to produce recombinantly. In order to overcome this challenge, Laminin-511 proteins were fragmented to find the smallest integrin-binding component and the E8 fragment was identified as the most promising result. In fact, hES cells were found to adhere more strongly to the E8 fragment than to the full-length protein.
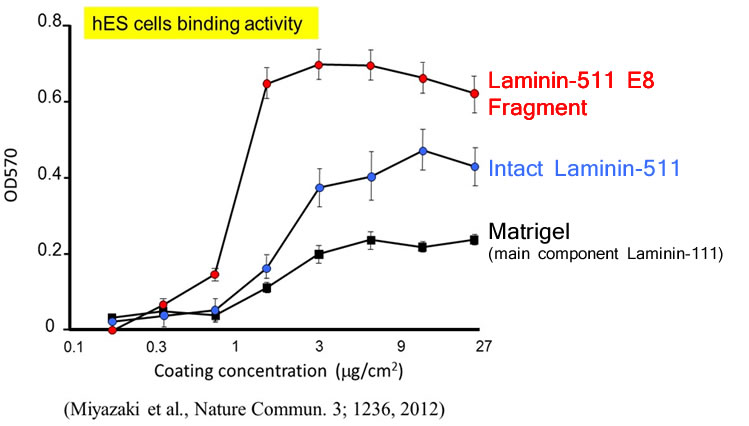
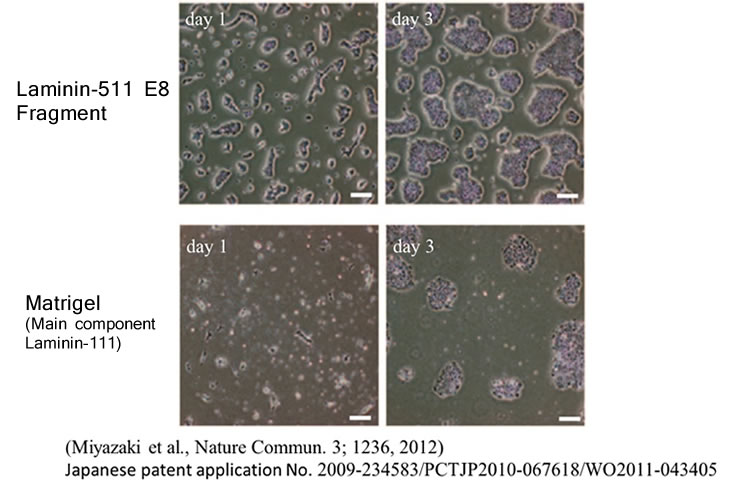
In the same study cited above, Human ES cells were passaged on Laminin-511 E8 fragment and other cell culture substrates. Upon singly dispersing human ES cell colonies at the time of passage, it was confirmed that single cells adhere rapidly to Laminin-511 E8 and proliferate immediately.
Reference Literature
1. Miner, JH and Yurchenco, PD Laminin functions in tissue morphogenesis. Annu. Rev. Cell Dev. Biol. 20, 255-284 (2004). https://doi.org/10.1146/annurev.cellbio.20.010403.094555
2. Ido H, Harada K, Futaki S, Hayashi Y, Nishiuchi R, Natsuka Y, Li S, Wada Y, Combs AC, Ervasti JM and Sekiguchi K. Molecular dissection of the alpha-dystroglycan- and integrin-binding sites within the globular domain of human laminin-10. J. Biol. Chem. 279, 10946-10954 (2004). https://doi.org/10.1074/jbc.M313626200
3. Ido H, Nakamura A, Kobayashi R, Ito S, Li S, Futaki S and Sekiguchi K. The requirement of the glutamic acid residue at the third position from the carboxyl termini of the laminin gamma chains in integrin binding by laminins. J. Biol. Chem. 282, 11144-11154 (2007). https://doi.org/10.1074/jbc.M609402200
4. Taniguchi Y, Ido H, Sanzen N, Hayashi M, Sato-Nishiuchi R, Futaki S and Sekiguchi K. The C-terminal region of laminin beta chains modulates the integrin binding affinities of laminins. J. Biol. Chem. 284, 7820-7831 (2009). https://doi.org/10.1074/jbc.M809332200
5. Miyazaki T, Futaki S, Suemori H, Taniguchi Y, Yamada M, Kawasaki M, Hayashi M, Kumagai H, Nakatsuji N, Sekiguchi K and Kawase E. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat. Commun. 3, 1236 (2012). https://doi.org/10.1038/ncomms2231
6. Nakagawa M, Taniguchi Y, Senda S, Takizawa N, Ichisaka T, Asano K, Morizane A, Doi D, Takahashi J, Nishizawa M, Yoshida Y, Toyoda T, Osafune K, Sekiguchi K, Yamanaka S. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. SCIENTIFIC REPORTS | 4 : 3594 | doi: 10.1038/srep03594
7. Yasuhiro Takashima, Ge Guo, Remco Loos, Jennifer Nichols, Gabriella Ficz, Felix Krueger, David Oxley, Fatima Santos, James Clarke, William Mansfield, Wolf Reik, Paul Bertone, Austin Smith Resetting Transcription Factor Control Circuitry toward Ground-State Pluripotency in Human. Cell Volume 158, Issue 6, p1254–1269, 11 September 2014 doi: 10.1016/j.cell.2014.08.029
8. Doi D et al. (2014). Isolation of Human Induced Pluripotent Stem Cell-Derived Dopaminergic Progenitors by Cell Sorting for Successful Transplantation. Stem Cell Reports. 2 (3): 337-50. https://doi.org/10.1016/j.stemcr.2014.01.013
9. Takashima Y et al. (2014). Resetting Transcription Factor Control Circuitry toward Ground-State Pluripotency in Human. Cell. 158 (6): 1254-69. https://doi.org/10.1016/j.cell.2014.08.029
10. Fukuta M et al. (2014). Derivation of mesenchymal stromal cells from pluripotent stem cells through a neural crest lineage using small molecule compounds with defined media. PLos One. 9 (12) : e112291. https://doi.org/10.1371/journal.pone.0112291
11. Hayashi et al. (April 2015). Structure-based discovery of NANOG variant with enhanced properties to promote self-renewal and reprogramming of pluripotent stem cells. PNAS vol. 112 (15): 4666-4671. https://doi.org/10.1073/pnas.1502855112
FOR RESEARCH USE ONLY, NOT FOR USE IN DIAGNOSTIC PROCEDURES.
Manufactured by : Nippi, Inc.
| Questions about this product? Email us at orders@iwai-chem.com or give us a call: (650) 486-1541 |
Product Videos
Custom Field
Product Reviews
1 Review Hide Reviews Show Reviews
-
imatrix-511
We have used this on variety of stem cells and works perfectly each time. It is a perfect substitute for cell cultures where collagen or fibronectin is needed as adhering agent. It is easy and quick to use and doesn't need to be incubated to activated.






